Needle-Trap wins new product award at the Scottish Business Awards 2013
Needle-Trap, a labeling option featuring a safety mechanism integrated as a component of the self-adhesive syringe label, has been named in the Scottish Business Awards as New Product of the Year.
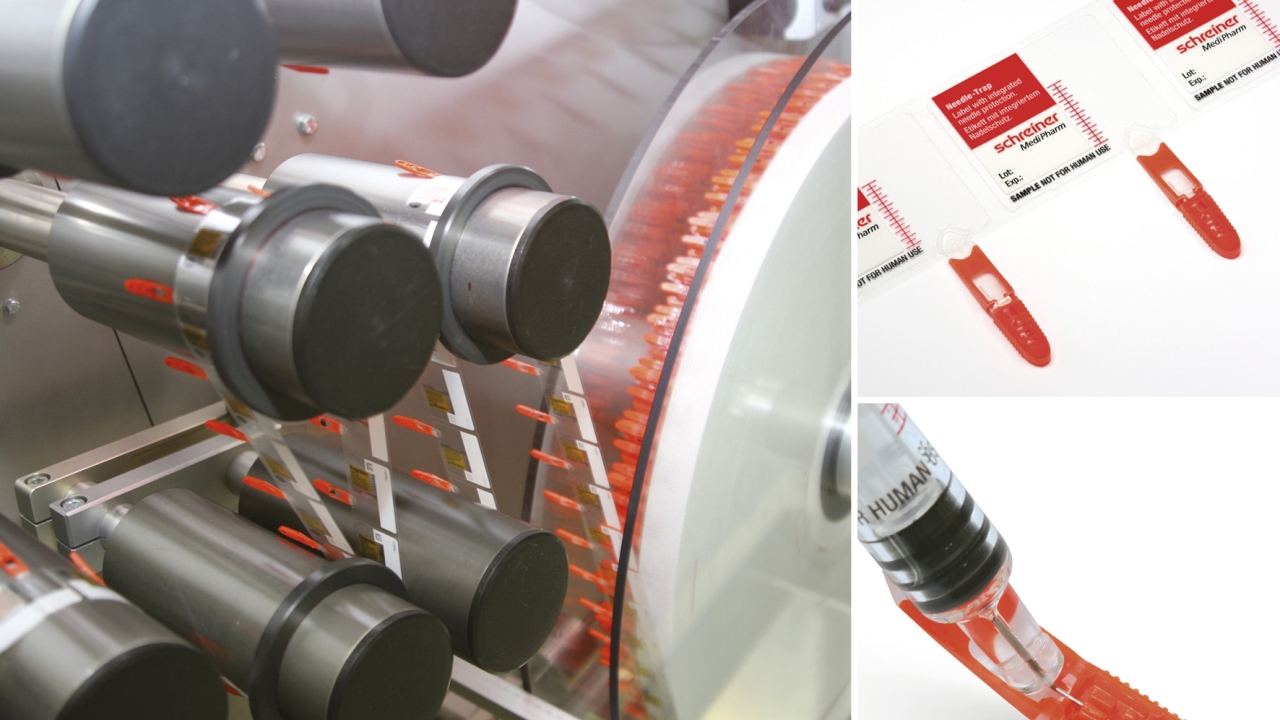
A product developed by pharma labeling system provider Schreiner MediPharm, Needle-Trap was nominated for the award by Scotland-based film specialist FLEXcon.
The Needle-Trap syringe label combines an injection-molded part with a film from FLEXcon, and protects the needles of pre-filled syringes after injection.
The user simply has to bend the needle trap that is integrated in the label towards the side before performing the injection. The system is activated by using one hand, and handling of the syringe remains unchanged. The process of securing the needle after the injection is 100 percent controllable and, unlike some automatic passive activation mechanisms, occurs without patient contact.
Schreiner MediPharm and FLEXcon worked together when selecting the material, surface coating, adhesive and liner material for the Needle-Trap product according to the requirements of its application.
Due to its use on clear containers, visual clarity is a crucial aspect of the film, and the contents of syringes must be visible after the label’s application.
The label’s surface properties are designed to make the material suitable for conventional printing and subsequent variable marking techniques. A high tack force ensures reliable adhesion to glass syringes and the needle trap itself, which is composed of plastic.
Further functionality, such as graduations or counterfeit protection features, may be integrated into the label. Needle-Trap also allows reliable documentation of injections that have been performed. For this purpose, the label is equipped with optional detachable label parts which healthcare personnel apply directly to the patient’s medical file or vaccination card.
The syringe label meets the requirements of the regulatory authorities for safety and quality, including the new EU Directive 2010/32/EU, and has been awarded 510(k) certification by the US Food and Drug Administration (FDA).
Lee Macnamara, application development and marketing director for FLEXcon Europe, said: ‘With our product we’re offering Schreiner MediPharm a customized solution that is tailored exactly to meet the needs of the application.’
Gene Dul, president of Schreiner MediPharm US, said: ‘Needle-Trap can be adapted to all commonly used syringe dimensions. Due to the simple design, the specialty labels can be easily processed on conventional labeling equipment.’
Stay up to date
Subscribe to the free Label News newsletter and receive the latest content every week. We'll never share your email address.

