The critical role of cryogenic labels in modern science
Find out how cryogenic labels are preserving the precious data in an environment where temperatures drop as low as -196 deg C.

In the quiet, climate-controlled heart of a modern laboratory, a small vial holds the potential for a cure, a new diagnostic tool or a lifeline for a patient. Within a biobank, millions of such vials are stored, each containing a unique and irreplaceable biological sample. These samples are preserved at the most extreme of temperatures, often in the vapor phase of liquid nitrogen at a chilling -196 deg C. At this frontier of scientific preservation, the integrity of a tiny, unassuming component is non-negotiable: the cryogenic label. Cryogenic labels are highly engineered products that must withstand conditions that would cause a standard sticker to crack, peel or fade into illegibility within minutes.
The failure of a cryogenic label is not a mere inconvenience. It can lead to the misidentification or loss of a sample, compromising years of research, invalidating a clinical trial, or jeopardizing patient safety. As the fields of personalized medicine, cell and gene therapy and biobanking expand at an exponential rate, the cryogenic label has transformed from a simple accessory into a foundational component of the scientific and medical cold chain.
The growth of the cryo market
The demand for reliable cryogenic labels is a direct reflection of the surging industries they support. According to a market analysis by Grand View Research, the global cryogenic label market was valued at 1.12 billion USD in 2024 and is projected to reach 2.15 billion USD by 2034, exhibiting a compound annual growth rate (CAGR) of 6.64 percent. This growth is not uniform but driven by specific, high-stakes applications.
As Paavo Sillanpää, senior manager at UPM Adhesive Materials, notes: ‘The cryogenic label market has enjoyed a healthy growth rate in recent years due to an increase in the number of biobanks, advances in research for biological drugs, cell therapies, genomics and regenerative medicine.’

Other industry experts echo this sentiment. Gabriela Gregor, senior marketing manager, pharma and specialty, Avery Dennison, shares: ‘We have seen an increased number of requests for labels used for cryogenic applications over the past years. It started during Covid-19 as the delivery of vaccines required low temperatures. Since then, the ultra-low temperature products have been steadily rising due to the advancement in biologic and genetic therapeutics.’
The science behind cryogenic labels
When selecting a cryogenic label, several key features are paramount to ensuring long-term success.
It goes without saying that labels must be certified to perform reliably at the full range of cryogenic temperatures, from -20 deg C (standard freezer), to -80 deg C (ultra-low temperature freezer), to the ultimate challenge of -196 deg C (liquid nitrogen).
“The primary challenge is achieving sufficient adhesion level at such a low temperature. A crucial aspect of this is the wide temperature range a label must endure."
The labels must also be able to withstand exposure to common laboratory solvents without smudging or peeling. What makes a label capable of surviving a liquid nitrogen bath? It is a complex, engineered system where every component is chosen for its ability to function under extreme duress. This is often achieved through a combination of the right facestock, a robust thermal transfer ribbon, cryogenic adhesives and a protective topcoat.
Adhesion at sub-zero temperatures
The adhesive is arguably the most critical component. A standard adhesive loses its tackiness and becomes rigid below its glass transition temperature. In this temperature range, a polymer-based adhesive undergoes a transition from a hard, brittle, glassy state to a soft, flexible, rubbery state or vice versa. It can’t conform to the shrinking surface of a vial, causing it to peel or delaminate. Cryogenic adhesives are formulated to remain flexible and functional at extremely low temperatures. Most cryogenic adhesives are specialized acrylics formulated for this purpose.
A key challenge is the difference in thermal contraction between the label material and the surface to which it’s applied, such as a polypropylene vial. The label and the vial will shrink at different rates, creating immense stress on the adhesive bond. A properly engineered cryogenic adhesive possesses a specific degree of flexibility that allows it to accommodate this movement without peeling or losing its grip.
As Gregor of Avery Dennison points out: ‘The primary challenge is achieving sufficient adhesion level at such a low temperature. A crucial aspect of this is the wide temperature range a label must endure, from application at room temperature to storage at -196 deg C, and in some cases, cryogenic cycles and thawing in a water bath at body temperature.’
For Soichiro (Sushi) Fujinaga, technical manager at Lintec, the chemistry behind these adhesives is proprietary information and a closely guarded secret. However, he states: ‘The performance hinges on a specialized formulation that allows the adhesive to accommodate the thermal expansion and contraction differences between the label and the container.’
The unbreakable facestock
In cryogenic applications, the facestock is far from a simple piece of paper. The material must be able to endure extreme cold without becoming brittle, cracking or losing its ability to hold ink. It must also resist chemical solvents, abrasion and moisture, all of which are common in a laboratory setting.
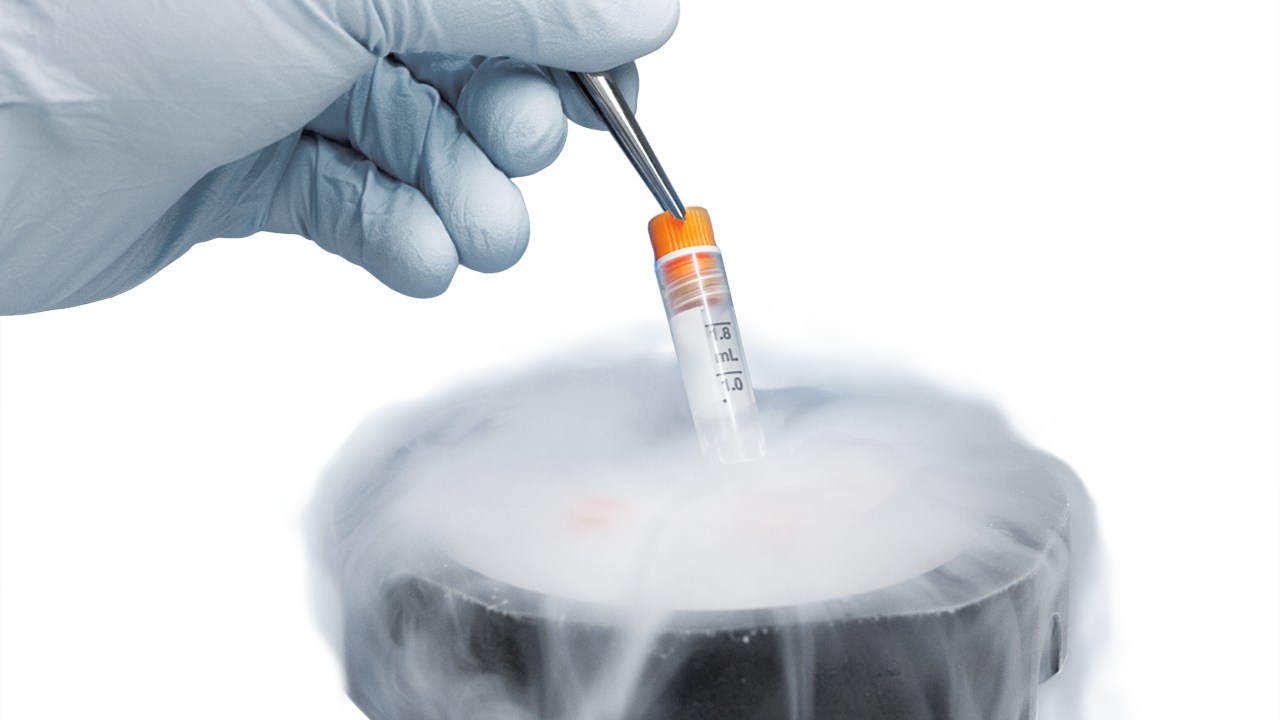
Standard paper and vinyl face stocks are generally not recommended for use in cryogenic applications. Paper, being porous, absorbs moisture, which then freezes and can cause the label to delaminate. Vinyl becomes brittle and loses its flexibility in low temperatures. This has led label manufacturers to adopt a range of specialized synthetic fi lms that offer superior performance.
Polyester is the most common material for cryogenic label face stocks due to its exceptional dimensional stability, thermal resistance and durability. It is a strong, rigid film that resists temperature-induced shrinkage and maintains its mechanical strength even at -196 deg C. Polyester’s chemical resistance makes it ideal for environments where labels may be exposed to harsh solvents like ethanol, isopropanol or xylene. It is also highly compatible with thermal transfer printing, which is standard for creating durable, smudge-proof labels. Gregor indicates that polyester films are ‘rigid and less conformable than polyethylene, making it ideal for flat and semi-rigid surfaces where labels must remain secure for years.’
Polyolefin films, such as polypropylene (PP) and polyethylene (PE), are primarily chosen for their conformability and flexibility. This is particularly important for small-diameter vials and tubes, as a flexible face stock can more easily wrap around a curved surface without flagging or peeling at the edges. Gregor further notes that ‘although PP and PE have better resistance to freezing and thawing, they may not be as reliable as polyester for long-term storage or the most extreme cryogenic conditions.’
While less discussed, the liner is essential for protecting the adhesive before application. A good liner ensures the adhesive remains pristine and ready to form a secure bond with the substrate.
Printing best practices
The most advanced label is useless if the information printed on it is unreadable. This has made thermal transfer printing among the most suitable techniques for cryogenic labels.
This method uses a heated printhead to melt a solid resin-based ribbon onto the face stock. ‘At UPM, we always recommend using a resin ribbon with our cryogenic label materials. Resin ribbons are designed to withstand extreme cold and chemical exposure,’ shares Sillanpää. ‘The resulting print is exceptionally durable and resistant to common lab solvents and abrasion.’
While TTR is dominant, other technologies are also used. Fujinaga mentions: ‘We also sell desktop laser printable and water-based inkjet versions for cryogenic labels.’
Gregor from Avery Dennison notes that ‘each technology has trade-offs; for instance, direct thermal printers do not use a ribbon, which can be an advantage if you want to ensure no personal information is left on a ribbon that needs to be securely disposed of.’
Even with the best materials, an improperly applied label will fail. Material manufacturers emphasize that a label requires ‘a clean surface with sufficient pressure and dwell time to adhere properly.’
Sillanpää notes that they ‘always recommend attaching labels in room temperature, if possible.’
Gregor echoes the same sentiment, stating: ‘We primarily print at room temperature and ensure long enough dwell time in order to let the adhesive bond with the substrate before cryogenic storage.’
“Each technology has trade-offs.”
The consensus among experts is that applying a label to a frozen, frosted, or wet vial is a major risk factor for failure. Brien Christopherson, vice president, research and development at Brady Corporation, a Milwaukee, US-based manufacturer of labels, signs, safety devices, printing systems and software, notes: ‘The biggest mistake we see is when customers try to apply our labels to vials that have already been frozen. It’s a natural instinct, but it’s crucial that the adhesive has a chance to form a strong initial bond before the temperature plummets.’
Trying to apply a standard label to a frosted vial will result in immediate and catastrophic failure, as moisture and temperature differences prevent any bond from forming. However, some specialized adhesives do exist. As Sillanpää notes: ‘Specialized cryo adhesives also allow labeling in sub-zero temperatures.’ For containers with small diameters, Gregor recommends an ‘overlap for test tubes in order to prevent label lifting.’
The crucial applications
The demand for high-performance cryogenic labels is inextricably linked to the rapid advancements in the medical and scientific fields. In these environments, every sample is a potential source of a cure, a vital piece of evidence or a key to understanding a disease.

In the medical field, cryogenic labels are widely used in biobanking. The global biobanking market is projected to reach over 182 billion USD by 2034, growing at a CAGR of 8.16 percent. Biobanks are vast repositories that store millions of biological samples such as blood, plasma, tissue and DNA for future research. The National Institute of Health in the US notes that in a large-scale biobank with millions of samples, one cannot afford a single mix-up. The entire value of a biobank is in its ability to retrieve and identify a sample decades later. The label is the most important part of that chain of custody.
The integrity of these labels is fundamental to the long-term viability of personalized medicine, which relies on accessing patient-specific biological data. Cryogenic labels are also used in clinical diagnostics, where patient samples need to be labeled with absolute precision to ensure accurate test results. The need for a label that can withstand rapid freezing and thawing cycles and resist common lab chemicals is non-negotiable for patient safety and diagnostic reliability.
Another area of application is in cell and gene therapy. The global cell and gene therapy market is projected to reach 117.46 billion USD by 2034, and each of these treatments requires meticulous labeling to ensure the right patient receives the right therapy.
Corinna Endres, product manager, drug protection at Schreiner MediPharm, states: ‘Due to the rise in novel therapies such as cell and gene, mRNA, etc, there has been a growing demand regarding cryogenic labels.’ These therapies, such as CAR-T cell therapy, use a patient’s own living cells. These precious, one-of-a-kind samples are frozen and stored in liquid nitrogen during transport and processing. A labeling error could have devastating consequences, rendering the therapy unusable or, in a worst-case scenario, leading to a patient receiving the wrong treatment. The label here is not just an identifier; it is a direct safeguard of the patient’s life.
Other than the medical arena, cryogenic labels play a major role in genomics and proteomics. Research labs that perform high-throughput screening may process thousands of samples a day. Automation and robotics are critical, and they depend on scannable barcodes on labels that are reliable and will not fail. The failure of a single label can disrupt an entire research workflow, leading to costly delays and the potential loss of irreproducible data.
Drug discovery is also an area where cryo labels are gaining popularity. Pharmaceutical companies rely on cryogenic storage to preserve vast libraries of chemical compounds and reagents. The ability to accurately track and retrieve specific compounds is essential to the lengthy and expensive process of discovering new drugs.
No room for error
The critical nature of cryogenic labels becomes painfully clear when they fail. A patient sample mix-up or loss can have catastrophic consequences, as documented by organizations like the Agency for Healthcare Research and Quality (AHRQ) and the Center for Phlebotomy Education. These agencies conclude that mislabeled specimens are a frequent problem, resulting in serious consequences, including the need for additional treatment or temporary or permanent harm to the patient.
The cost of these errors is staggering. The College of American Pathologists (CAP) estimates that a single mislabeled specimen costs an institution about 712 USD. For a large hospital, the cost of mislabeling can exceed one million USD per year.
The challenges of the cold chain are not static, and neither are the solutions. The market is constantly evolving to meet new demands.
Gregor highlights the growing need for tamper-evident labels for secondary packaging, such as cardboard boxes and plastic storage boxes. This adds another layer of security to the cold chain, ensuring samples have not been compromised during transport or storage. Fujinaga, too, notes: ‘There’s the constant need to develop new products to match our customers’ requirements as clinical technologies like syringes and test tubes evolve.’
The ultimate goal for many in the industry is automated tracking. The integration of RFID or NFC chips into cryogenic labels would allow for the simultaneous scanning of entire racks of samples without ever needing to open a freezer.
‘RFID/NFC have already been integrated into cryogenic labels for tracking, but there are significant challenges with extremely low temperature ranges and reliable functionality of the integrated chip,’ comments Endres. ‘The thermal shock can cause stress on the chip and wire bonds, leading to failure. However, many companies are actively working to overcome these hurdles. We, at Schreiner MediPharm, have advanced test capabilities to verify that RFID readability is possible even at minus 80 deg C.’
“The biggest mistake we see is when customers try to apply our labels to vials that have already been frozen. It's a natural instinct.”
RFID cryogenic labels are engineered to withstand extreme conditions, remaining scannable even when submerged in liquid nitrogen at -196 deg C. These labels, provided by manufacturers such as US-based AssetPulse, feature a flexible UHF inlay and permanent adhesive securely identifying vials and tubes in long-term cryogenic storage. Unlike traditional barcodes, passive RFID labels can be read without direct line-of-sight, reducing the need for freeze–thaw cycles that risk sample integrity. This consistent and reliable tracking minimizes errors, while also supporting strict regulatory compliance in sensitive environments such as hospitals, where RFID is used to access the right sample for life-saving procedures.
Similarly, Identiv and InPlay are co-developing a portfolio of BLE-enabled smart labels for high-value logistics, including cold-chain, pharmaceuticals and food. Powered by InPlay’s ultra-lowpower IN100 NanoBeacon SoC, the labels provide real-time tracking, visibility, and condition monitoring (temperature, humidity, motion). With extended read ranges, smartphone compatibility, and built-in AES-EAX encryption, the labels are designed for secure, scalable use in specialized environments such as cryogenic logistics. Commercial launch for these labels is planned for late 2025.
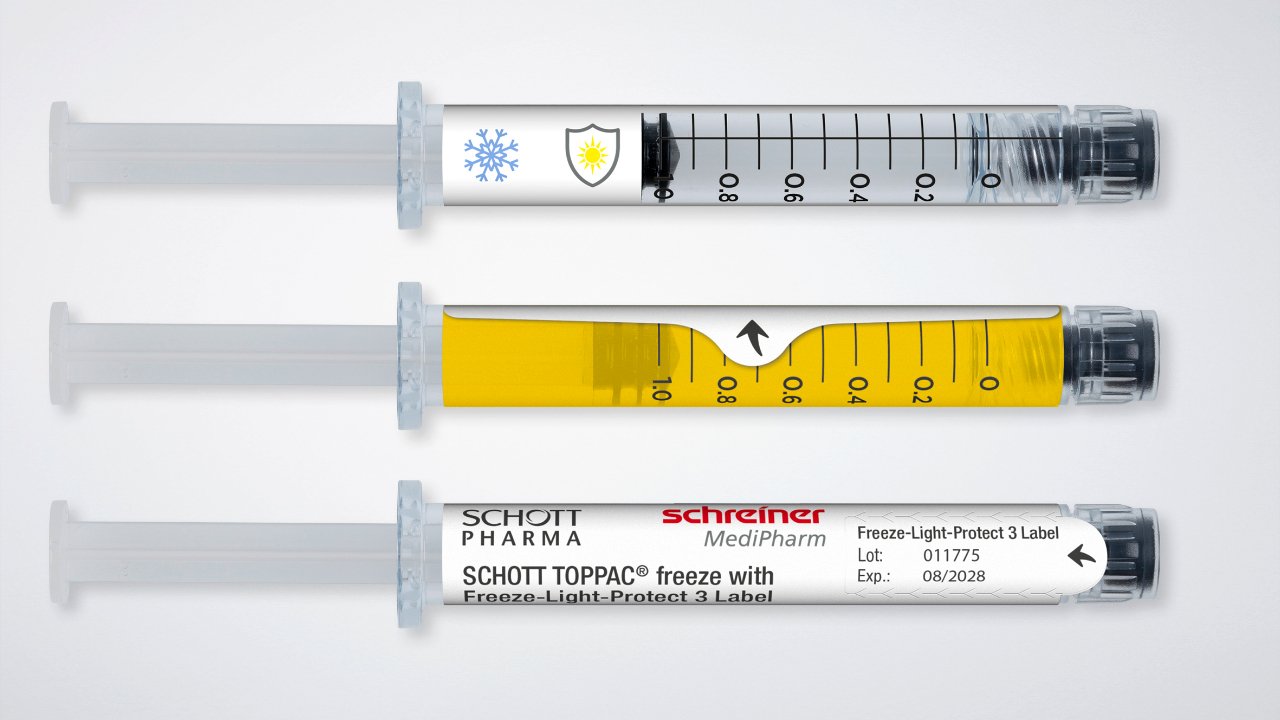
Cryogenic labels are no longer just for vials. Schreiner MediPharm has developed a Freeze-Light-Protect label to address the challenge of light-sensitive drugs. This innovative solution combines cryogenic durability with multi-level UV and light protection. This is critical for biologics and other sensitive substances stored in transparent containers, where maintaining drug efficacy is just as important as maintaining sample identification.
Small label with a monumental impact
Cryogenic labels are a hidden but indispensable part of modern science and medicine. They are not mass-produced commodity items but highly specialized, engineered solutions designed to function under the most punishing conditions imaginable. From the microscopic scale of a single cryovial to the massive scale of a national biobank, these labels are the silent guardians of integrity, ensuring that a sample collected today can be reliably identified and used a decade from now.
The market for these labels is thriving, fueled by unprecedented growth in fields that rely on the preservation of biological materials. As cell therapies become more patient-centric and personalized medicine moves into the mainstream, the need for reliable, secure, and smart labeling will only increase.
Stay up to date
Subscribe to the free Label News newsletter and receive the latest content every week. We'll never share your email address.


