Schreiner MediPharm introduces UV and light protection labels
Schreiner MediPharm, a Germany-based global provider of specialty pharmaceutical labeling products, has introduced labels that protect liquid medicines in transparent glass containers from damaging UV and light exposure.
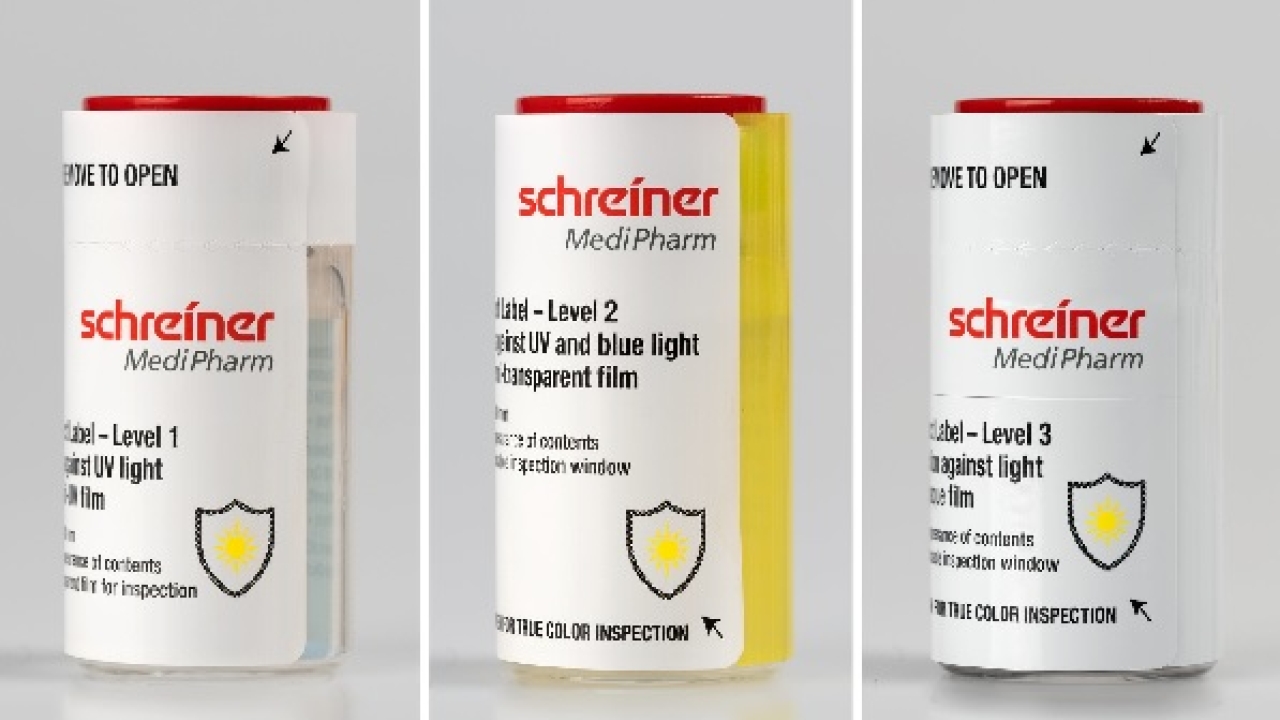
The company’s newest portfolio includes three protective labels at various safety levels that safeguard against UV and light, while also allowing caregivers and users to ensure the liquid inside is in its intended, original condition.
Light-sensitive substances are often filled into brown glass vials to protect them against UV irradiation and light. In that scenario, an inherent disadvantage is that the original color of the preparation is no longer discernible, and the medicine cannot be visually inspected for particles or color changes before dispensing. Conversely, while transparent glass containers permit liquids to be inspected, they allow light and UV rays to pass through.
The specialty labels from Schreiner MediPharm for UV and light protection are designed for three safety levels: Level 1 labels have a transparent inspection window with UV protection. At level 2, the labels are equipped with a semi-transparent, colored window and a reclosable inspection window that protects against UV rays and blue light. Level 3 labels consist of an opaque label material for complete light protection; an additionally integrated, reclosable inspection window allows for viewing the container content.
All three label concepts are adapted to the specific requirements of the respective substance, the individual containers as well as to UV and light requirements. Manufacturers of biologics and other sensitive products are therefore able to effectively protect highly sensitive substances against light and UV irradiation, avoiding potential health risks for patients due to medicines that have been damaged by light.
Stay up to date
Subscribe to the free Label News newsletter and receive the latest content every week. We'll never share your email address.

