Schreiner MediPharm develops Flexi-Cap
Schreiner MediPharm has developed Flexi-Cap, a label and cap combination anti-counterfeiting system that features a first-opening indicator designed to prevent the illegal reuse of medicine containers under the guise of being unopened, original products.
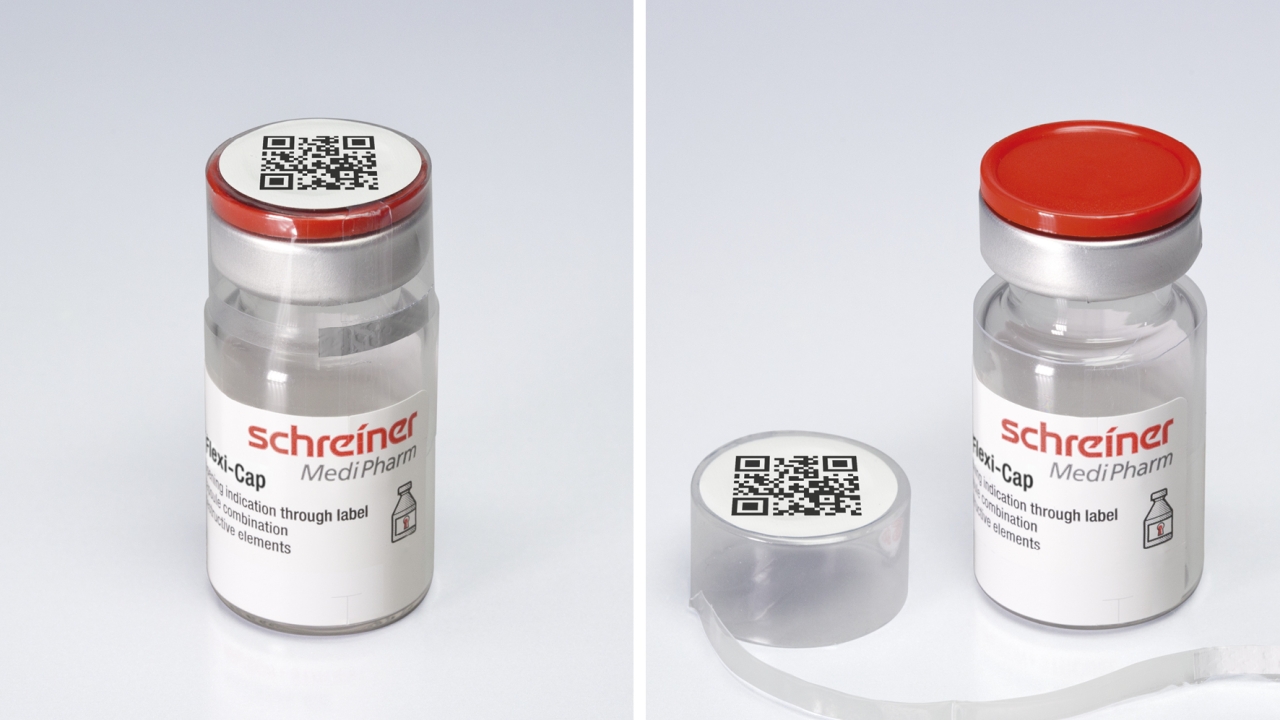
Flexi-Cap comprises a label combined with a cap – similar to the cover for wine bottles but adapted to the specific requirements of the pharmaceutical industry.
The film cap is positioned over the closed container before the label is applied without covering the peel-open tab of the opening strip. Once the strip is opened, the bottom part of the cap, together with the label, remains attached to the container. Attempting to remove the rest of the cap destroys the label, eliminating the possibility of unnoticed illegal reuse, Schreiner MediPharm.
Flexi-Cap can be integrated into existing production processes, and augmented by adding extra security features such as holographs, color-shifting inks, void effects or the machine-readable LaserSecure technology.
Flexi-Cap enables flexible use with different container types, forms and sizes. In contrast to shrink-wrap solutions, the label construction can be applied without using heat, making the system viable for temperature-sensitive medicines.
The pharmaceutical manufacturer's label and brand design remain unchanged, due to the combination of label and cap. In addition, the top of the cap allows space for barcode printing or NFC chip integration for electronic tracking.
Gene Dul, president of Schreiner MediPharm US, said: ‘Flexi-Cap’s innovative security concept allows for flexible, customized implementation of a highly effective anti-counterfeiting tactic.
‘It accomplishes this by yielding plainly apparent physical and visual evidence of a container’s use – ones that vastly diminish the potential for illicit market reintroduction.’
Stay up to date
Subscribe to the free Label News newsletter and receive the latest content every week. We'll never share your email address.

