Avery Dennison extends pharmaceutical portfolio with syringe labeling line
Avery Dennison has introduced a line of new syringe labeling products to its pharmaceutical portfolio featuring safe, compliant labelstocks that meet manufacturers’ high standards for syringe and tight mandrel pharmaceutical applications.
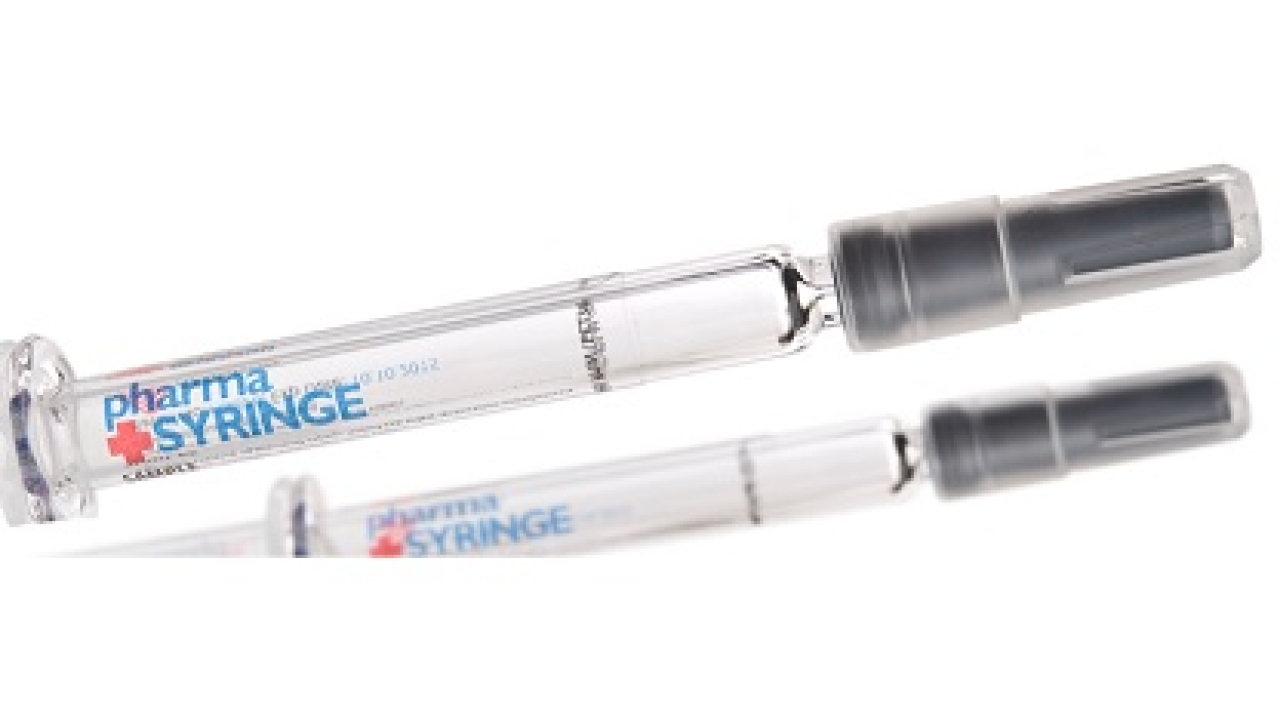
The new products include low migration and high-performance adhesives on flexible paper and film facestocks. Products are sterilization-friendly and also resistant to heat, steam and chemicals. Photoluminescent options are available to aid with missing label detection using ultraviolet light.
‘According to the IMS Institute for Healthcare Informatics, the global pharmaceutical segment is projected to grow to more than one trillion USD by 2018, with focused growth in the oncology, diabetes, pain and autoimmune therapy areas,’ said David Collins, North America marketing director for specialty products at Avery Dennison Label and Packaging Materials. High value drugs used to treat the aforementioned ailments are commonly packaged in pre-filled syringes or vials and require labels that will remain securely in place during storage and use, and function according to the needs of the product. Further, adhesives used within the pharmaceutical industry must undergo comprehensive FDA testing to ensure they comply with toxicology requirements. If any adhesive changes are to occur, Avery Dennison will notify converters months in advance in order to ensure new formulations are properly tested for compliance.
Stay up to date
Subscribe to the free Label News newsletter and receive the latest content every week. We'll never share your email address.

