Domino launches serialization technology for pharma
Domino Printing Sciences has launched K600G, a new blister foil and web digital printing technology for product serialization in pharmaceutical applications.
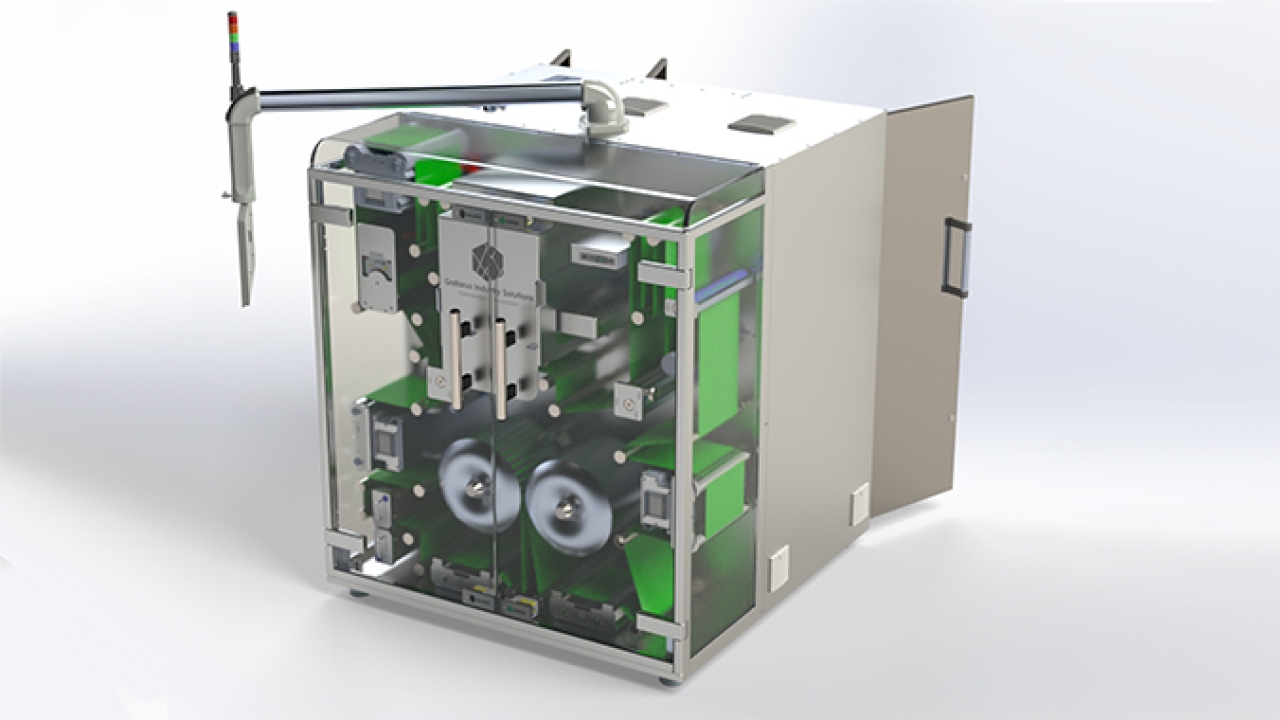
The new technology has been developed in collaboration with pharmaceutical industry innovator Gallarus and engagement from life science industry experts SeaVision.
‘Over the last couple of years, serialization at pack level has become a global requirement in the pharmaceutical industry,’ said Craig Stobie, director of global sector development at Domino. ‘In the future, it is likely that serialization of individual pockets of a blister pack will become the new standard in order to further enhance patient safety and reduce medical errors.
‘The K600G has been developed to provide manufacturers with an on-site solution for variable data printing, which includes coding at the item level, to meet these future requirements.’
The new K600G includes a range of options developed for different installation types, including off-line, near-line, top-of-line, sealed-blister-coding for printing directly on formed blister packs, and an in-line version designed to meet the needs of OEMs.
‘Digital printing and flexible supply chains are beginning to play a much greater role in pharmaceutical manufacturing – and so the K600G is a necessary investment to keep up with future legislation and market trends,’ added Volker Watzke, EU medical devices sector manager at Domino. ‘As Domino customers, pharmaceutical manufacturers utilizing the K600G solution also have the benefit of our global experts, who are always ready to support them when they need us. We want to ensure that our customers are always in control of their lines.’
When it comes to printing performance, the K600G achieves reliable printing across a range of substrates with a native resolution of 600DPI and greyscale capability. It is based on Domino’s piezo drop-on-demand inkjet technology. It can build up imagery using multiple different drop sizes to improve image quality and give manufacturers more control over their ink consumption.
‘In pharmaceutical manufacturing, code quality and legibility are of key importance, and, owing to the cost of unplanned downtime, it is also imperative that machines are kept functioning at optimal performance,’ commented Bart Vansteenkiste, global sector manager for life sciences at Domino. ‘The K600G was developed with this level of reliability and durability in mind with automated maintenance systems to reduce operator intervention and keep the system running at optimal performance.’
The system can be supplied with unique AI software that collects, analyses, and learns from factory data to provide intelligent manufacturing technologies.
‘With the K600G, we have combined the key principles of Industry 4.0 to provide a solution that addresses the evolving life science environment and production challenges facing pharmaceutical manufacturers,’ said John McKeon, CEO of Gallarus. ‘The new solution delivers exponential benefits in the form of ROI, cost-saving, quality enhancement, and OEE efficiencies.’
The K600G is capable of printing at speeds of up to 75 m/min, and print widths range from a single print module, covering 108mm (4.25in), up to seven dual print modules with a combined width of 782mm (30.81in). The smart i-Tech StitchLink micro-motor provides accurate print head alignment and image stitching to achieve seamless printing across a full web print width.
The i-Tech CleanCap function cleans the print heads when not in operation, leaving them wiped and capped, ready for the next use. This removes the need for daily print head cleaning, protects them from blockage and damage, and reduces the need for maintenance.
‘When we set out to develop the K600G, we wanted to develop a future-proof solution that would meet the current needs of pharmaceutical manufacturers and allow them to adapt more easily to changing market trends and legislative requirements,’ said Watzke. ‘The result is a strong and reliable solution that helps to ensure compliance and patient safety while allowing manufacturers to remain agile. When it comes to pharmaceutical manufacturing, the K600G is the solution of choice, now and in the future.’
Stay up to date
Subscribe to the free Label News newsletter and receive the latest content every week. We'll never share your email address.

